Articles
- Page Path
- HOME > J Musculoskelet Trauma > Volume 33(3); 2020 > Article
- Review Article Current Concepts of Bone Healing
- Dong Hun Suh, Bong Mo Koo, Jong Woo Kang
-
Journal of Musculoskeletal Trauma 2020;33(3):171-177.
DOI: https://doi.org/10.12671/jkfs.2020.33.3.171
Published online: July 31, 2020
- 2,306 Views
- 91 Download
- 0 Crossref
- 0 Scopus
Abstract
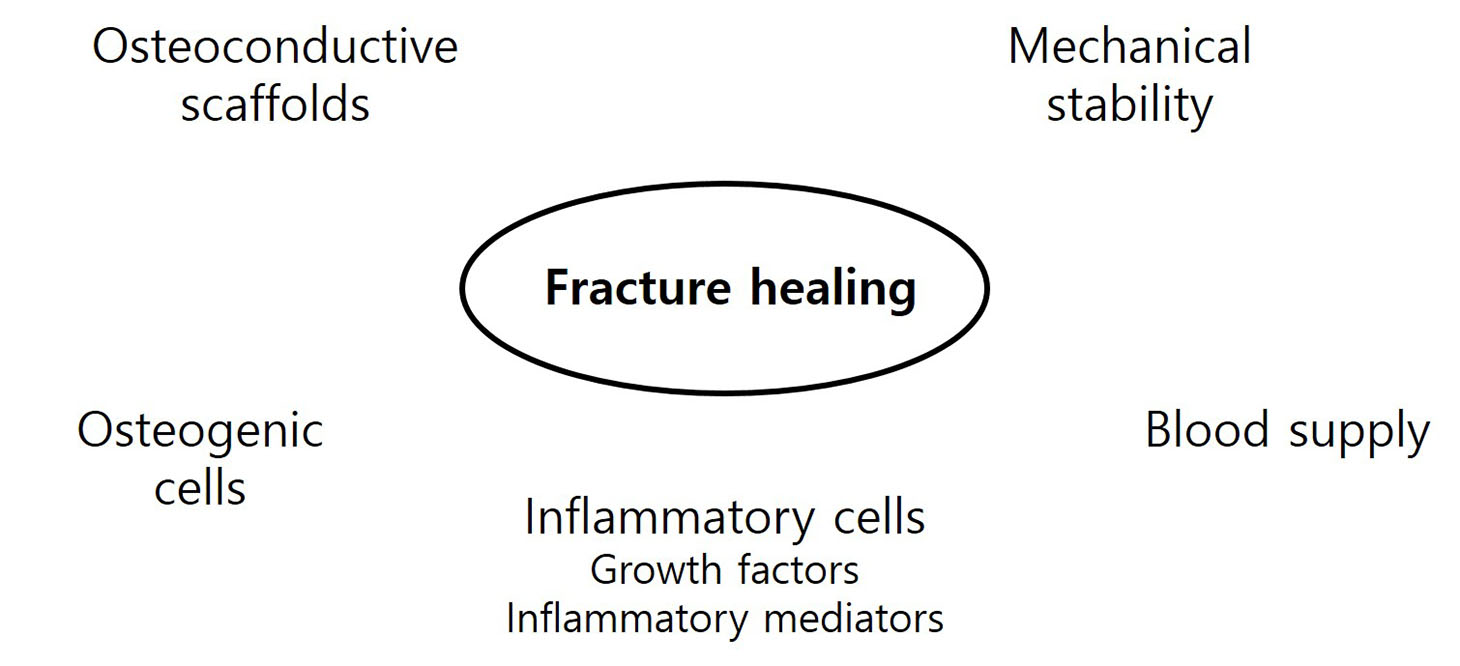
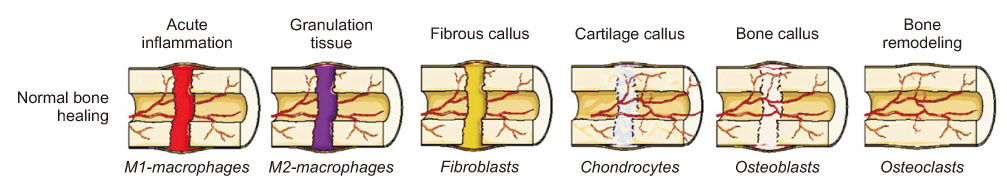
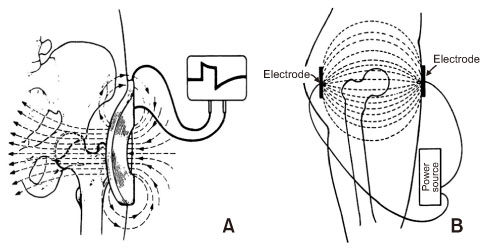
Bone injuries induce an inflammatory response that promotes bone healing. On the other hand, an aberrant process, where inflammation becomes chronic, can inhibit the healing of injured bone. At the first stage of the bone healing process, inflammatory cells, such as neutrophils and macrophages, are assembled and secrete various cytokines, chemokines, and growth factors. During callus formation, cells differentiated from mesenchymal stem cells, such as osteoblasts and chondrocytes, play leading roles in bone healing. Currently, various treatment modalities have been developed through the known mechanism of bone healing, and the clinical outcomes of bone defect and fracture nonunion have been good.
Published online Jul 24, 2020.
https://doi.org/10.12671/jkfs.2020.33.3.171
Current Concepts of Bone Healing
Abstract
Bone injuries induce an inflammatory response that promotes bone healing. On the other hand, an aberrant process, where inflammation becomes chronic, can inhibit the healing of injured bone. At the first stage of the bone healing process, inflammatory cells, such as neutrophils and macrophages, are assembled and secrete various cytokines, chemokines, and growth factors. During callus formation, cells differentiated from mesenchymal stem cells, such as osteoblasts and chondrocytes, play leading roles in bone healing. Currently, various treatment modalities have been developed through the known mechanism of bone healing, and the clinical outcomes of bone defect and fracture nonunion have been good.
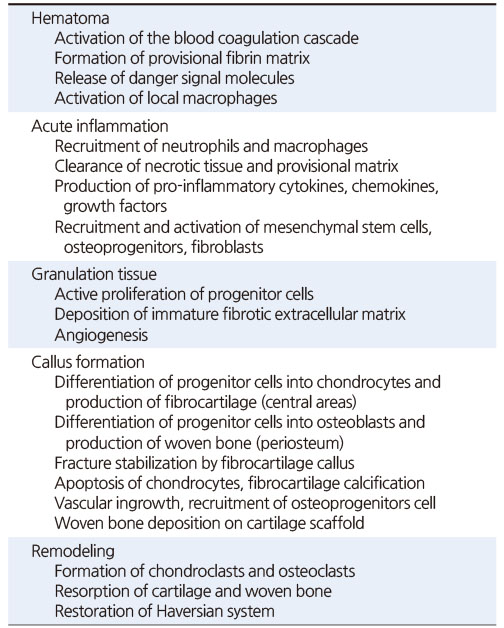
Table 1
Key Events during Secondary Fracture Healing
Financial support:None.
Conflict of interests:None.
References
-
Miranda MA, Moon MS. Treatment strategy for nonunions and malunions. In: Stannard JP, Schmidt AH, Kregor PJ, editors. Surgical treatment of orthopaedic trauma. New York: Thieme; 2007. pp. 77-100.
-
-
National Osteoporosis Foundation. What is osteoporosis and what causes it? [Internet]. Arlington: National Osteoporosis Foundation; [cited 2020 Apr 15].Available from: https://www.nof.org/patients/what-
is- osteoporosis/.
-

 E-submission
E-submission KOTA
KOTA TOTA
TOTA TOTS
TOTS


 Cite
Cite